Background: We previously reported thatearly events in follicular lymphoma (FL), defined as progression of disease within 24 months (POD24) or early transformation to a more aggressive histology, are significantly associated with increased risk of lymphoma related death. To refine clinical management, the FLIPI24 prognostic model was developed to predict the risk of early events after starting immunochemotherapy (IC) (Maurer et al, 2022). The FLIPI24 model was found to be significantly superior to standard clinical models for prediction of both event-free survival at 24 months (EFS24) and overall survival (OS) in internal and external validation sets of patients receiving IC. We sought to evaluate the prognostic ability of the FLIPI24 model in patients with FL not receiving IC.
Methods: The cohort consists of prospectively enrolled patients in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER) and the multicenter Lymphoma Epidemiology of Outcomes (LEO) Cohorts. Data were collected from medical records by standard protocol with all events verified by medical record review. Eligible patients were those diagnosed with grades 1-3A FL and initiated observation, rituximab monotherapy, radiation, or other non-IC therapy. The FLIPI24 model utilizes 5 continuous variables: age (linear 60-90 years with inflection at age 75), HGB (linear 8-17 g/dL), WBC (linear 4-11x10 9/L), LDH/ULN (linear 0.5-5), and B2M (linear 1-10 mg/L). FLIPI24 risk is grouped as follows: low risk (≤10%], low-average risk (10-15%], average risk (15-20%], high risk (20-40%], very high risk (>40%) for an early event. EFS was defined as time from diagnosis to progression, relapse, (re)treatment, histologic transformation, or death due to any cause. OS was defined as the time from diagnosis until death from any cause. Cox models and Kaplan Meier curves were used to evaluate association between FLIPI24 and EFS or OS.
Results: 1542 patients initiating non-IC management approaches at diagnosis from 2002-2020 were identified. 51% were >60 years (IQR 52-70), and 49% were male. Most patients (86%) had FL grade 1-2. 61% of patients met any criteria for high tumor burden per GELF, BLNI, or GITMO based on available variables. Treatment approaches were 833 observation (54%), 315 rituximab monotherapy (20%), 186 radiation (12%), and 208 other non-IC approaches (14%). At median follow-up of 58 months, EFS24 was 73.8% (95% CI: 71.6%, 76.1%), 5-year OS was 90.9% (95% CI: 89.3%, 92.5%) and 253 (16%) patients died.
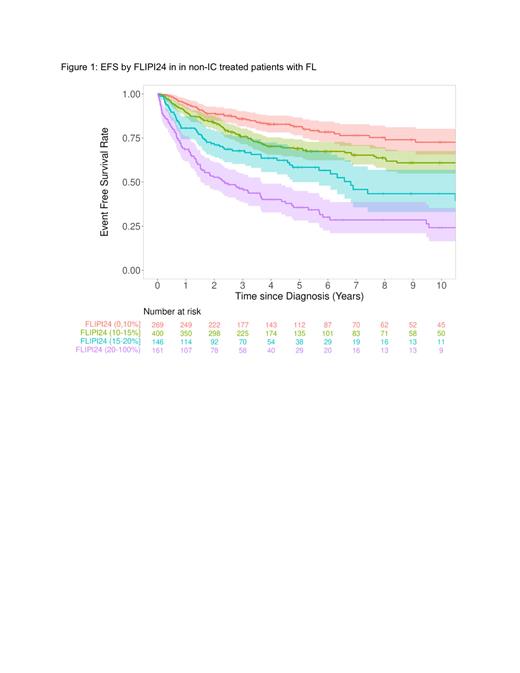
The FLIPI24 score identified poor outcomes among patients with high/very high-risk score (FLIPI24 >20%), with median EFS of 1.8 years (95% CI, 1.3, 3.28) and 5 year OS of 65.1% (95% CI: 55.9-75.8) (Figure 1). When evaluating patient subsets, for patients with high tumor burden (defined based on available variables) and high/very high-risk score, median EFS was 1.6 years and 5 year OS was 64.6% (95% CI: 54.9%-76.1%). Among patients with low tumor burden FL and high/very high-risk score, median EFS was 2.0 years (95% CI: 0.84-NA) and 5 year OS was 66.2% (95% CI: 42.4%-100%). Patients undergoing observation with high/very high-risk score similarly had shortened median EFS of 1.53 years (95% CI: 1.25-NA) and 5 year OS of 68.9% (95% CI: 56.4%-84.1%).
The FLIPI24 had superior concordance for OS (c=0.726) compared to the FLIPI (c=0.646) when evaluated in patients not treated with IC. Results were comparable in the subsets of patients observed (c=0.706 vs 0.596) and receiving rituximab monotherapy (c=0.764 vs 0.721).
Conclusion: The FLIPI24 model has been previously validated to provide an individual risk score at diagnosis for the likelihood of experiencing an event within 24 months from starting IC. Here we demonstrate that a high FLIPI24 score predicts for poorer outcomes following non-IC strategies including observation, treatment with radiation, and rituximab monotherapy. The FLIPI24 has better prognostic performance for OS than FLIPI in this non-IC population. High FLIPI24 in patients not treated with IC identifies a patient population who should be considered for IC and/or novel frontline therapies when feasible.
Disclosures
Casulo:Follicular Lymphoma Foundation: Other: Leadership role; Gilead Sciences: Research Funding; SecuraBio: Research Funding; Abbvie: Consultancy; Verastem: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; GenMab: Research Funding; Genentech: Consultancy, Research Funding; Lymphoma Research Foundation: Other: Leadership Role. Flowers:Xencor: Research Funding; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Spectrum: Consultancy; Genentech Roche: Consultancy, Research Funding; Denovo Biopharma: Consultancy; TG Therapeutics: Research Funding; V Foundation: Research Funding; Burroghs Wellcome Fund: Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Iovance: Research Funding; Gilead: Consultancy, Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Genmab: Consultancy; Cancer Prevention and Research Institute of Texas: Research Funding; National Cancer Institute: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Ziopharm: Research Funding; 4D: Research Funding; Morphosys: Research Funding; Jannsen Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding; Nektar: Research Funding; Karyopharm: Consultancy; SeaGen: Consultancy; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; Kite: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Amgen: Research Funding; Pharmacyclics Jansen: Consultancy; Celgene: Consultancy, Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Habermann:Genentech: Research Funding; sorrento: Research Funding; BMS: Research Funding. Witzig:Kura Oncology: Research Funding; Karyopharm: Research Funding; ADC: Membership on an entity's Board of Directors or advisory committees; Salarius Pharma: Membership on an entity's Board of Directors or advisory committees. Wang:Genmab: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Novartis: Research Funding; Morphosys: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Nowakowski:Zai Lab Limited: Consultancy; Selvita Inc: Consultancy; Abbvie: Consultancy; Incyte: Consultancy; MEI Pharma: Consultancy; Seagen: Consultancy; Genentech: Consultancy; Kymera Therapeutics: Consultancy; Kite Pharma: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Bantam Pharmaceutical LLC: Consultancy; Blueprint Medicines: Consultancy; Celgene Corporation: Consultancy; Debiopharm: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Curis: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Regeneron: Honoraria; AstraZeneca: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Cohen:BMS/Celgene: Research Funding; Novartis: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Lossos:LRF: Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria; NCI: Research Funding; University of Miami: Current Employment; NCI: Research Funding; BeiGene: Consultancy. Kahl:BMS: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; ADCT: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Lilly: Consultancy, Honoraria. Cerhan:Protagonist: Other: Safety Monitoring Committee; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; NanoString: Research Funding; Genentech: Research Funding. Maurer:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Research Funding; BMS: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal